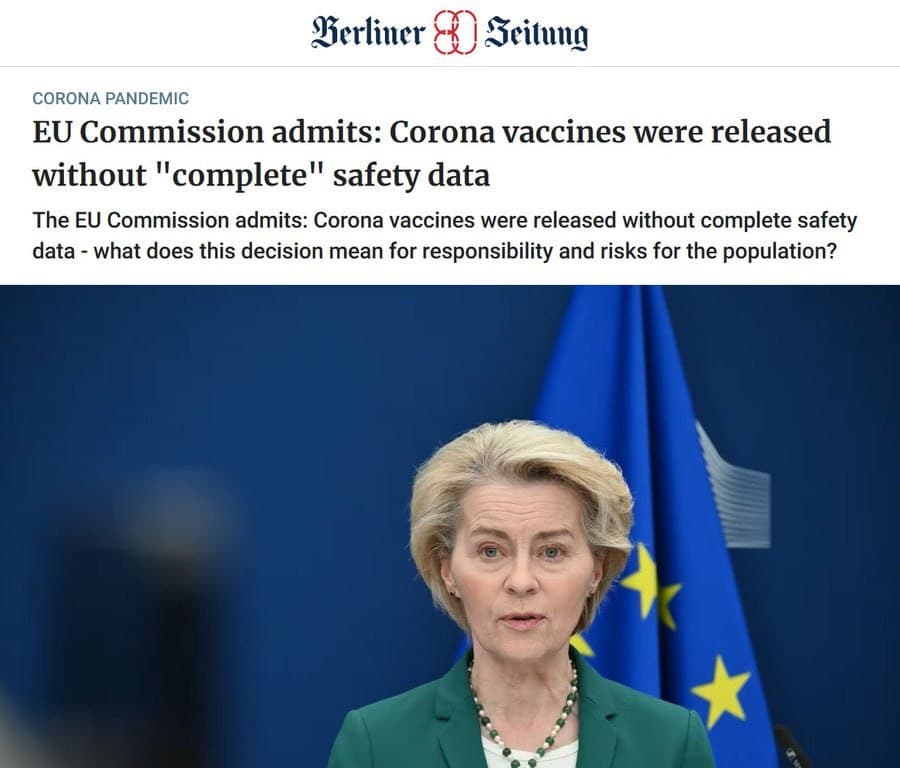
The European Commission has acknowledged that COVID-19 mRNA vaccines were authorized for human use without complete safety data. This admission marks a significant development in the ongoing discussion regarding the safety and efficacy of these vaccines.
As previously reported, the release of these vaccines was expedited during the pandemic, raising questions about the thoroughness of the safety evaluations conducted prior to their approval. The Commission"s recent statement highlights concerns that have been voiced by various stakeholders regarding the transparency of the vaccine approval process.
This revelation adds to the body of information surrounding the COVID-19 vaccination campaign and its regulatory framework. For further details, refer to the recent developments in this topic.






![[Video] Gunfire between Iraqi security forces and Sadr militias in Baghdad](/_next/image?url=%2Fapi%2Fimage%2Fthumbnails%2Fthumbnail-1768343508874-4redb-thumbnail.jpg&w=3840&q=75)
