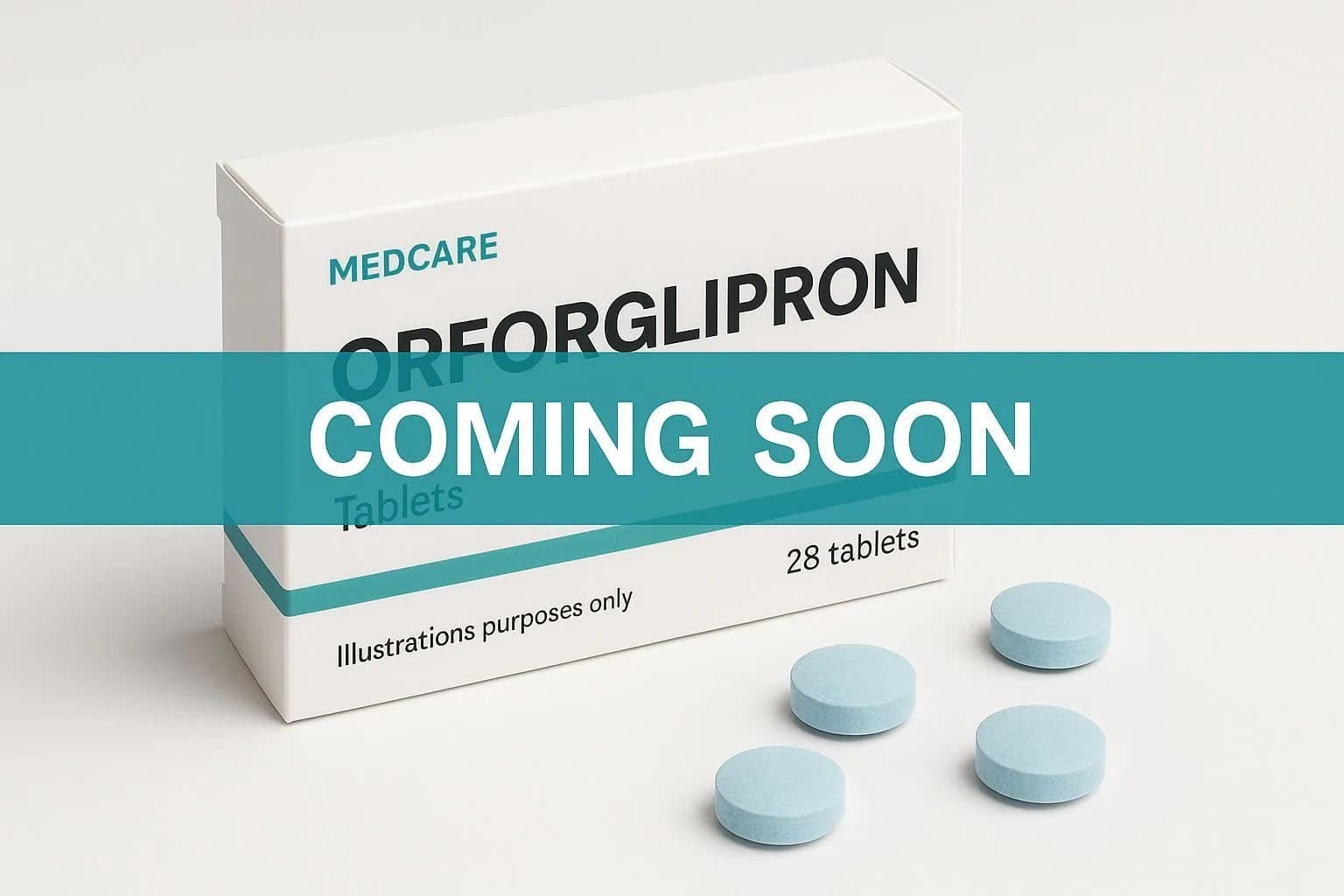
Pharmaceutical giant Eli Lilly has announced successful results from late-stage clinical trials for its weight loss drug, orforglipron, which demonstrated an average weight loss of 10.5%, or 22.9 pounds, among patients with obesity and type 2 diabetes over a 72-week period. This breakthrough positions the drug for potential regulatory approval globally.
Orforglipron, which is administered in tablet form, offers a significant advantage over existing weight loss treatments that require costly weekly injections. Notably, it also does not impose dietary restrictions, making it more accessible to a broader range of patients.
Eli Lilly is preparing to launch orforglipron on the global market, targeting a rollout "around the same time next year." This timeline indicates a growing urgency in addressing obesity, a condition affecting millions worldwide.
As obesity rates continue to rise, the development of effective treatments like orforglipron is critical. The positive trial results may reshape the landscape of weight loss medications, providing a more convenient option for those struggling with weight management. For further context, recent developments in health and medical research may shed light on similar situations impacting public health strategies.






![[Video] Gunfire between Iraqi security forces and Sadr militias in Baghdad](/_next/image?url=%2Fapi%2Fimage%2Fthumbnails%2Fthumbnail-1768343508874-4redb-thumbnail.jpg&w=3840&q=75)
